Buy Generic Tucatinib Online
Inquire NowTable of Contents
ToggleWhy Tucatinib prescribed?
Tucatinib is used with trastuzumab and capecitabine to treat a certain type of hormone receptor–positive breast cancer that has spread to other parts of the body and cannot be treated with surgery in adults who have already been treated with at least one other chemotherapy medication. Tucatinib is in a class of medications called kinase inhibitors. It works by blocking the action of the abnormal protein that signals cancer cells to multiply. This helps stop or slow the spread of cancer cells. To know more visit MedlinePlus.
Why should you buy the generic Tufactinib online?
First of all generic Tucaxen is very affordable version of Tucatinib for breast cancer patients. It is a verified generic version of Tukysa. Everest pharmaceuticals Limited manufacture this generic version in Bangladesh. It is one of the renowned pharmaceutical companies who mostly made cancer medicine in Bangladesh. After the Directorate General of Drug Administration of Bangladesh approves to manufacture Tucatinib , they started to make it and sell it all over the world where generic medicine is allowed to use.
It is the first generic version of Tukysa which is selling regularly all over the world. It is also 83% cheaper than the brand version of Tucatinib. Which cost you less than 900 dollars. There are some more medicine available for breast cancer patients. Palbociclib is one of them. To know more you can visit our shop page.
COMPOSITION
TUCAXEN 50 TABLET: Each film-coated tablet contains Tucatinib INN 50 mg.
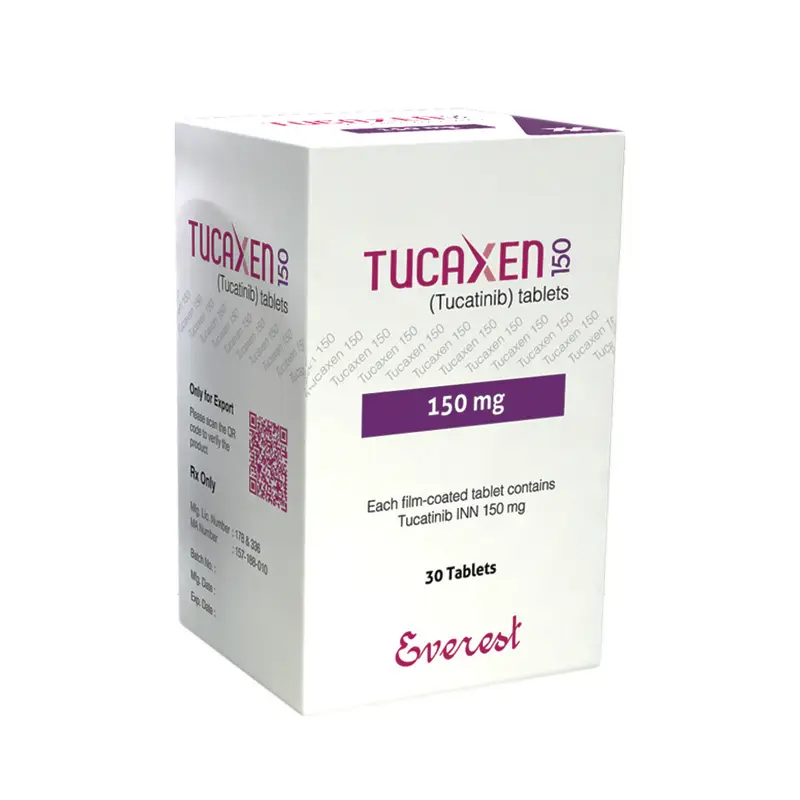
TUCAXEN 150 TABLET: Each film-coated tablet contains Tucatinib INN 150 mg.
PHARMACOLOGY OF TUCATINIB
Mechanism of Action
Tucatinib is a tyrosine kinase inhibitor of HER2. In vitro, tucatinib inhibits phosphorylation of HER2 and HER3, resulting in inhibition of downstream MAPK and AKT signaling and cell proliferation, and showed anti-tumor activity in HER2 expressing tumor cells. In vivo, tucatinib inhibited the growth of HER2 expressing tumors. The combination of tucatinib and trastuzum ab showed increased anti-tumor activity in vitro and in vivo compared to either drug alone.
Pharmacodynamics
Exposure Response Relationship
Tucatinib exposure-response relationships and the time course of pharmacodynamics response have not been fully characterized.
Cardiac Electrophysiology
No large mean increase in OTc (i.e., > 20 ms) was detected following treatment with Tucatinib at the recommended dose of 300 mg taken orally twice daily.
Pharmacokinetics
Tucatinib AUCO-INF and Cmax increases proportionally over a dosage range from 50 mg to 300 mg (0.17 to 1 times the approved recommended dosage). Tucatinib exhibited 1.7-fold accumulation for AUC and 1.5-lold accumulation for Cmax following administration of Tucatinib 300 mg twice daily for 14 days. Time to steady state was approximately 4 days.
Absorption
The median time to peak plasma concentration of Tucatinib was approximately 2 hours (range 1 to 4 hours).
Effects of Food
Following administration of a single oral dose of Tucatinib in 11 subjects alter a high-lat meal (approximately 58% lat, 26% carbohydrate, and 16% protein), the mean AUCO-INF increased by 1.5-lold, the Tmax shifted from 1.5 hours to 4 hours, and Cmax was unaltered. The effect of food on the pharmacokinetics of tucatinib was not clinically meaningful.
Distribution
The geometric mean (CV%) apparent volume of distribution of tucatinib was approximately 1670 L (66%). The plasma protein binding was 97.1% at clinically relevant concentrations.
Elimination
The geometric mean (CV%) hall-life of tucatinib was approximately 8.5 (21%) hours and apparent clearance was 148 Uh (55%).
Metabolism
Tucatinib is metabolized primarily by CYP2C8 and to a lesser extent via CYP3A.
Excretion
Following a single oral dose of 300 mg radiolabeled Tucatinib, approximately 86% of the total radiolabeled dose was recovered in feces (16% of the administered dose as unchanged tucatinib) and 4.1% in urine with an overall total recovery ol 90% within 13 days post-dose. In plasma, approximately 76% of the plasma radioactivity was unchanged, 19% was attributed to identified metabolites, and approximately 5% was unassigned.
INDICATION
Tucatinib is indicated in combination with trastuzumab and capecitabine for treatment of adult patients with advanced unresectable or metastatic HER2-positive breast cancer, including patients with brain metastases, who have received one or more prior anti-HER2-based regimens in the metastatic setting.
DOSAGE AND ADMINISTRATION
Recommended Dosage
The recommended dosage of Tucatinib is 300 mg taken orally twice daily in combination with trastuzumab and capecitabine until disease progression or unacceptable toxicity.
Patients are advised to swallow Tucatinib tablets whole and not to chew, crush, or split prior to swallowing. Patients are advised not to ingest tablet if it is broken, cracked, or not otherwise intact.
Patients are advised to take Tucatinib approximately 12 hours apart and at the same time each day with or without a meal.
II the patient vomits or misses a dose of Tucatinib, Patients are advised to take the next dose at its usual scheduled time.
When given in combination with Tucatinib, the recommended dosage of capecitabine is 1000 mg/m2 orally twice daily taken within 30 minutes alter a meal. Tucatinib and capecitabine can be taken at the same time.
Dosage Modifications for Adverse Reactions
Recommended Tucatinib Dose Reductions for Adverse Reactions
| Dose Reduction | Recommended Tucatinib Dosage |
| First | 250 mg orally twice daily |
| Second | 200 mg orally twice daily |
| Third | 150 mg orally twice daily |
Permanently discontinue Tucatinib in patients unable to tolerate 150 mg orally twice daily.
Dosage Modifications for Severe Hepatic Impairment
For patients with severe hepatic impairment (Child-Pugh C), reduce the recommended dosage to 200 mg orally twice daily.
Dosage Modifications for Concomitant Use with Strong CYP2C8 Inhibitors
Avoid concomitant use of strong CYP2C8 inhibitors with Tucatinib. II concomitant use with a strong CYP2C8 inhibitor cannot be avoided, reduce the recommended dosage to 100 mg orally twice daily. Alter discontinuation of the strong CYP2C8 inhibitor for 3 elimination hall-lives, resume the Tucatinib dose that was taken prior to initiating the inhibitor.
CONTRAINDICATION
None.
WARNING AND PRECAUTION
Diarrhea
Tucatinib can cause severe diarrhea including dehydration, hypotension, acute kidney injury, and death. In HER2CLIMB, 81% of patients who received Tucatinib experienced diarrhea, including 12% with Grade 3 diarrhea and 0.5% with Grade 4 diarrhea. Both patients who developed Grade 4 diarrhea subsequently died, with diarrhea as a contributor to death. The median time to onset of the first episode of diarrhea was 12 days and the median time to resolution was 8 days. Diarrhea led to dose reductions of Tucatinib in 6% of patients and discontinuation of Tucatinib in 1% of patients. Prophylactic use of antidiarrheal treatment was not required on HER2CLIMB.
II diarrhea occurs, administer antidiarrheal treatment as clinically indicated. Perform diagnostic tests as clinically indicated to exclude other causes of diarrhea. Based on the severity of the diarrhea, interrupt dose, then reduce dose or permanently discontinue Tucatinib.
Hepatotoxicity
Tucatinib can cause severe hepatotoxicity .In HER2CLIMB, 8% of patients who received Tucatinib had an ALT increase > 5 x ULN, 6% had an AST increase > 5 x ULN, and 1.5% had a bilirubin increase > 3 x ULN (Grade ;o:3) Hepatotoxicity led to dose reduction of Tucatinib in 8% of patients and discontinua tion of Tucatinib in 1.5% of patients.
Monitor ALT, AST, and bilirubin prior to starting Tucatinib, every 3 weeks during treatment, and as clinically indicated. Based on the severity of hepatotoxicity, interrupt dose, then reduce dose or permanently discontinue Tucatinib.
SIDE EFFECTS
The following clinically significant side effects are observed:
- Diarrhea
- Hepatotoxicity
The most common side effects in patients who received Tucatinib (;o:20%) were diarrhea, palmar-plantar erythrodyses thesia, nausea, fatigue, hepatotoxicity, vomiting, stomatitis, decreased appetite, abdominal pain, headache, anemia, and rash.
USE IN SPECIFIC POPULATIONS
Pregnancy
Risk Summary
Tucatinib is used in combination with trastuzumab and capecitabine. Based on findings in animals and its mechanism of action, Tucatinib can cause fetal harm when administered to a pregnant woman .There are no available human data on Tucatinib use in pregnant women to inform a drug-associated risk. Advise pregnant women and females of reproductive potential of the potential risk to the fetus. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Lactation
Tucatinib is used in combination with trastuzumab and capecitabine. There are no data on the presence of Tucatinib or its metabolites in human or animal milk or its effects on the breastfed child or on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise women not to breastfeed during treatment with Tucatinib and for at least 1 week after the last dose.
Females and Males of Reproductive Potential
Tucatinib can cause fetal harm when administered to a pregnant woman Tucatinib is used in combination with trastuzumab and capecitabine.
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating treatment with Tucatinib.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with Tucatinib and for at least 1 week after the last dose.
Males
Male patients with female partners of reproductive potential are advised to use effective contraception during treatment with Tucatinib and for at least 1 week after the last dose.
Infertility
Based on findings from animal studies, Tucatinib may impair male and female fertility.
Pediatric Use
The safety and effectiveness of Tucatinib in pediatric patients have not been established.
Geriatric Use
In HER2CLIMB, 82 patients who received Tucatinib were ;,, 65 years, of whom 8 patients were ;,, 75 years. The incidence of serious adverse reactions in those receiving Tucatinib was 34% in patients ;,, 65 years compared to 24% in patients <65 years. The most frequent serious adverse reactions in patients who received Tucatinib and ;,, 65 years were diarrhea (9%), vomiting (6%), and nausea (5%). There were no observed overall differences in the effectiveness of Tucatinib in patients;,,65 years compared to younger patients. There were too few patients ;o:75 years to assess differences in effectiveness or safety.
Renal Impairment
The use of Tucatinib in combination with capecitabine and trastuzumab is not recommended in patients with severe renal impairment (Cler < 30 ml/min estimated by Cockcroft-Gault Equation), because capecitabine is contraindicated in patients with severe renal impairment.
No dose adjustment is recommended for patients with mild or moderate renal impairment (creatinine clearance [Cler] 30 to 89 ml/min).
Hepatic Impairment
Tucatinib exposure is increased in patients with severe hepatic impairment (Child-Pugh C). Reduce the dose of Tucatinib for patients with severe (Child-Pugh C) hepatic impairment.
No dose adjustment for Tucatinib is required for patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment.
OVERDOSE
There is no specific antidote, and the benefit of haemodialysis in the treatment of tucatinib overdose is unknown. In the event of an overdose, treatment with tucatinib should be withheld and general supportive measures should be applied.
PHARMACEUTICAL INFORMATION
Storage
Store below 25°C, in a cool and dry place. Keep away from light. Keep out of the reach of children.
How Supplied
TUCAXEN 50 TABLET: Each HOPE container contains 30 film-coated tablets (each tablet contains 50 mg Tucatinib) a silica gel desiccant and polyester coil with a child-resistant closure.
TUCAXEN 150 TABLET: Each HOPE container contains 30 film-coated tablets (each tablet contains 150 mg Tucatinib) a silica gel desiccant and polyester coil with a child-resistant closure.
Buy Generic Tucatinib 150 mg Online
Are you interested to buy tucatinib 150 mg online? Don’t worry. 100 Meds can help you to find reliable and verified medicine at affordable cost. Please contact our support team by using email, whatsapp or wechat. Even you can contact our facebook page.
You must be logged in to post a review.




Reviews
There are no reviews yet.